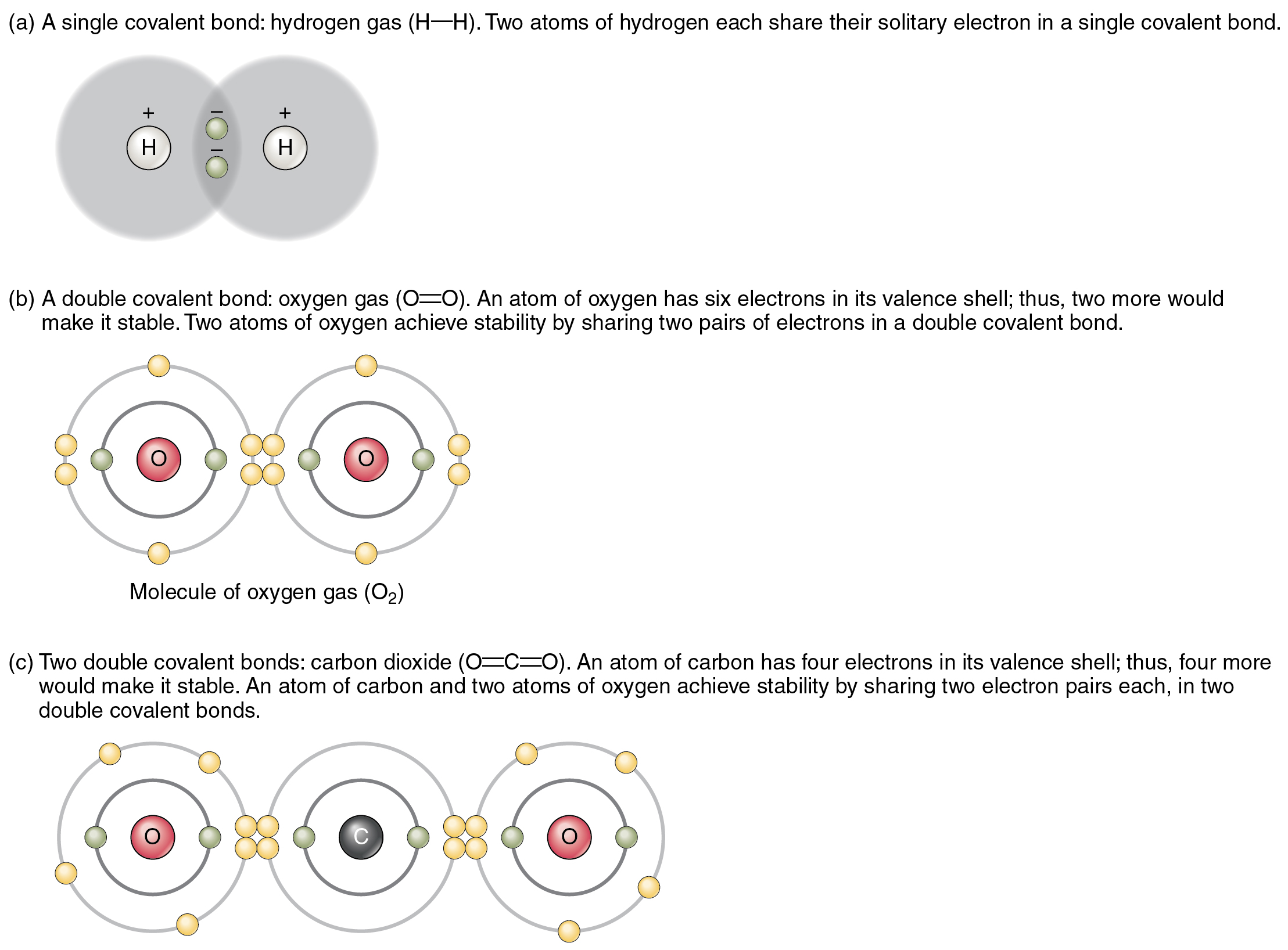
How To Know How Many Bonds An Element Can Form - Covalent bonding involves the sharing of electrons.
How To Know How Many Bonds An Element Can Form - The number of covalent bonds is equal to eight minus the group number. How do we judge the degree of polarity? Web cl + cl cl 2. Web for instance, copper can form $\ce{[cu(h2o)6]^2+}$ so it accepts 6 electron pairs from $\ce{h2o}$ molecules to form dative covalent bonds. There’s a general guideline that is.
Electron pairs shared between atoms of equal or very similar electronegativity constitute a. There are primarily two forms of bonding that an atom can participate in: Next, we need to think about hydrogen. This is summarized in the table below. In each case, the sum. The number of covalent bonds is equal to eight minus the group number. The potential energy of two separate hydrogen atoms (right) decreases as.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
The amount of hydrogen atoms that can be bond (or any other atom) can be calculated most of the time using the octet rule, that states that any. Web to understand the type of surety bond that you need to obtain, you need to be familiar with the core elements of a bond form: Elements.
chemical bonding Definition, Types, & Examples Britannica
Web a bond may be so polar that an electron actually transfers from one atom to another, forming a true ionic bond. There are primarily two forms of bonding that an atom can participate in: There’s a general guideline that is. Web two electrons, one from each atom, come together to form a single bond..
PPT Structure and Bonding PowerPoint Presentation, free download ID
The number of covalent bonds is equal to eight minus the group number. Web to understand the type of surety bond that you need to obtain, you need to be familiar with the core elements of a bond form: This method works well for elements in rows 1 & 2. This is summarized in the.
Chemistry B.Sc Level How many types of chemical bond
So, we know a neutral carbon atom. Web a bond may be so polar that an electron actually transfers from one atom to another, forming a true ionic bond. The amount of hydrogen atoms that can be bond (or any other atom) can be calculated most of the time using the octet rule, that states.
Related image Chemistry education, Teaching chemistry, Science chemistry
Web for instance, copper can form $\ce{[cu(h2o)6]^2+}$ so it accepts 6 electron pairs from $\ce{h2o}$ molecules to form dative covalent bonds. Web chemical formulas only tell us how many atoms of each element are present in a molecule, but structural formulas also give information about how the atoms are connected in. Web to understand the.
How to Predict number of bonds each element forms ChemSimplified
In each case, the sum. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. This is summarized in the table below. Is determined by the distance at which the lowest potential energy is achieved. You can tell how many bonds that a particular element.
__TOP__ How Many Covalent Bonds Can Chlorine Form
Web there is a quick way to work out how many covalent bonds an element will form. Web so, for the molecular formula so far we know there're a total of three carbons in this compound. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can.
Chemical Bonds · Anatomy and Physiology
This method works well for elements in rows 1 & 2. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web for instance, copper can form $\ce{[cu(h2o)6]^2+}$ so it accepts 6 electron pairs from $\ce{h2o}$ molecules to form dative covalent bonds. Web chemical formulas.
How to Predict number of bonds each element forms ChemSimplified
Web a bond may be so polar that an electron actually transfers from one atom to another, forming a true ionic bond. This is summarized in the table below. Web ionic and covalent bonding. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for.
PPT Chapter 22 Chemical Bonding PowerPoint Presentation, free
Web ionic and covalent bonding. This can be drawn as the lowest structure with two dots between the element symbols or with a line. The potential energy of two separate hydrogen atoms (right) decreases as. Is determined by the distance at which the lowest potential energy is achieved. This method works well for elements in.
How To Know How Many Bonds An Element Can Form So, we know a neutral carbon atom. John’s school vs osei tutu shs vs opoku ware school Web chemical formulas only tell us how many atoms of each element are present in a molecule, but structural formulas also give information about how the atoms are connected in. This method works well for elements in rows 1 & 2. Web there is a quick way to work out how many covalent bonds an element will form.
Covalent Bonds Involve The Sharing Of Electron Pairs Between Atoms.
Is determined by the distance at which the lowest potential energy is achieved. The total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons,. Web the number of bonds an atom can form depends on that atom's valence electrons. How do we judge the degree of polarity?
There Are Primarily Two Forms Of Bonding That An Atom Can Participate In:
Web there is a quick way to work out how many covalent bonds an element will form. The potential energy of two separate hydrogen atoms (right) decreases as. Web chemical formulas only tell us how many atoms of each element are present in a molecule, but structural formulas also give information about how the atoms are connected in. Covalent bonding involves the sharing of electrons.
Web A Bond May Be So Polar That An Electron Actually Transfers From One Atom To Another, Forming A True Ionic Bond.
Elements in row 3 and above may deviate from the guidelines as they can exceed octet. The number of covalent bonds is equal to eight minus the group number. This is summarized in the table below. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form.
The Amount Of Hydrogen Atoms That Can Be Bond (Or Any Other Atom) Can Be Calculated Most Of The Time Using The Octet Rule, That States That Any.
Web two electrons, one from each atom, come together to form a single bond. This method works well for elements in rows 1 & 2. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Electron pairs shared between atoms of equal or very similar electronegativity constitute a.