How To Draw Carbon Atom - Web 0:00 / 1:37 lewis dot structure of co2 (carbon dioxide) kentchemistry.com 25.1k subscribers 225k views 12 years ago every video i quickly take you through how.
How To Draw Carbon Atom - (valence electrons are the number of electrons present in the outermost shell of an atom). This video discusses the resonance structure of this polyatomic ion as well as the. Web for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Determine the total number of electrons in the carbon and oxygen valence shells. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1.
Add enough electrons (dots) to the outer atoms to. There are enough hydrogen atoms attached to each carbon to make the total number of bonds on that carbon up to 4. In the periodic table, oxygen belongs to the via group and has six electrons in its final shell. Web steps to draw the bohr model of carbon atom 1. Hydrogens that are attached to elements other than carbon are shown. Web we will use this information to draw the bohr model of the carbon atom. Web 0:00 / 1:37 lewis dot structure of co2 (carbon dioxide) kentchemistry.com 25.1k subscribers 225k views 12 years ago every video i quickly take you through how.
How To Draw Carbon at How To Draw
Take a pen and paper with you and try to draw this lewis structure along with me. Web for carbon, there are four valence electrons, two in the 2s subshell and two in the 2p subshell. For this, we will first have to calculate the number of protons and neutrons present in this atom. Add.
Carbon Atom Ascension Glossary
In the periodic table, oxygen belongs to the via group and has six electrons in its final shell. Web step 1: Because hydrogen only needs two. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. For this, we will first have to calculate.
Drawing Atoms Montessori Muddle
In the periodic table, oxygen belongs to the via group and has six electrons in its final shell. These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Web shorthand (line) formulas omit the symbols for carbon and.
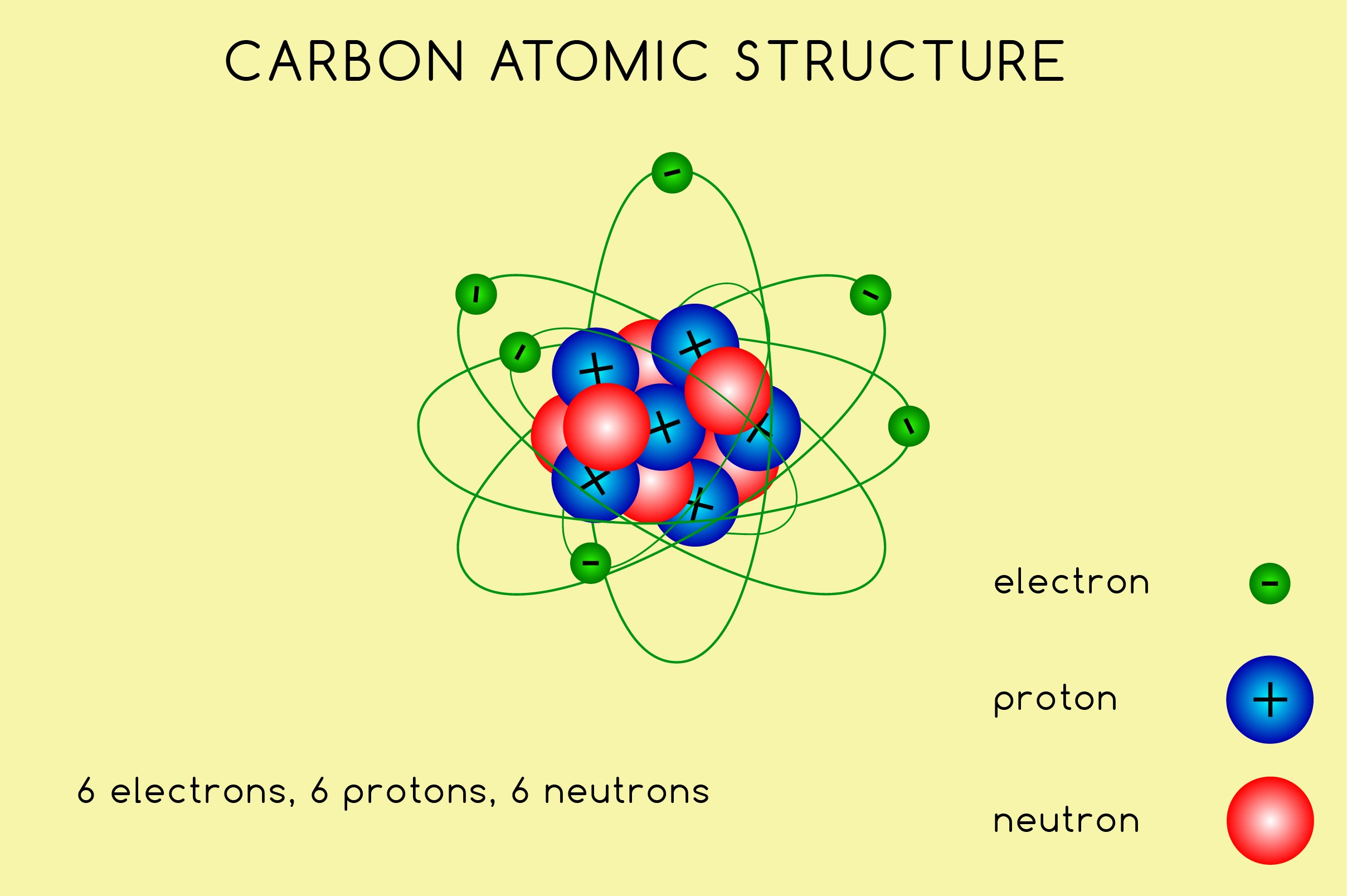
Carbon Atomic Structure High Resolution Stock Photography and Images
Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. Web 0:00 / 14:06 how to draw atoms (bohr model) highschoolscience101 36.1k subscribers 24k views 2 years ago chemistry in this lesson i present an overview of: Lone pair electrons are usually omitted. Because.
[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds
Web for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Let’s draw and understand this lewis dot structure step by step. For this, we will first have to calculate the number of protons and neutrons present in this.
Draw and label the structure of an atom of carbon Brainly.in
Calculation of valence electrons in co2 for carbon: Find the number of protons, electrons, and neutrons in the carbon atom protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Determine the total number of electrons in the carbon and oxygen valence shells. Web 0:00.
Electron configurations
So, let’s calculate this first. Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. 98k views 2 years ago. Calculation of valence electrons in co2 for carbon: Web a carbon atom is present wherever a line intersects another line. Questions tips & thanks.
Carbon atomic structure (437243) Illustrations Design Bundles
Web for example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. Web step 1: 98k views 2 years ago. Atom labels for all other elements are shown. Because hydrogen only needs two. So let's go back down and let's.
Carbon atom diagram concept Royalty Free Vector Image
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Atom labels for all other elements are shown. Web in order to draw the lewis structure of co2, first of all you have to find the total number of valence electrons present in the co2 molecule. Add enough.
Basic Chemistry Tutorial 2, Drawing Atoms sciencemusicvideos
Connect the atoms to each other with single bonds to form a “skeleton structure.” be sure that you follow rule 1. Web a carbon atom is present wherever a line intersects another line. Web 0:00 / 1:37 lewis dot structure of co2 (carbon dioxide) kentchemistry.com 25.1k subscribers 225k views 12 years ago every video i.
How To Draw Carbon Atom Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. Hydrogen atoms are omitted but are assumed to be present to complete each of carbon's four bonds. Let’s draw and understand this lewis dot structure step by step. For this, we will first have to calculate the number of protons and neutrons present in this atom. So let's go back down and let's calculate the total number of valence electrons that we need to represent in our dot structure.
However, Conventionally, We Draw The Dots.
So, let’s calculate this first. (valence electrons are the number of electrons present in the outermost shell of an atom). Web step 1: Hydrogens that are attached to elements other than carbon are shown.
Web For Carbon, There Are Four Valence Electrons, Two In The 2S Subshell And Two In The 2P Subshell.
For this, we will first have to calculate the number of protons and neutrons present in this atom. And oxygen is in group six, so six valence electrons for oxygen. Questions tips & thanks want to join the conversation? Web we will use this information to draw the bohr model of the carbon atom.
Figure Out How Many Electrons The Molecule Must Have, Based On The Number Of Valence Electrons In Each Atom.
98k views 2 years ago. Web how to draw human cell step by step. Because hydrogen only needs two. Web here's hydrogen, in group one, so one valence electron.
Add Enough Electrons (Dots) To The Outer Atoms To.
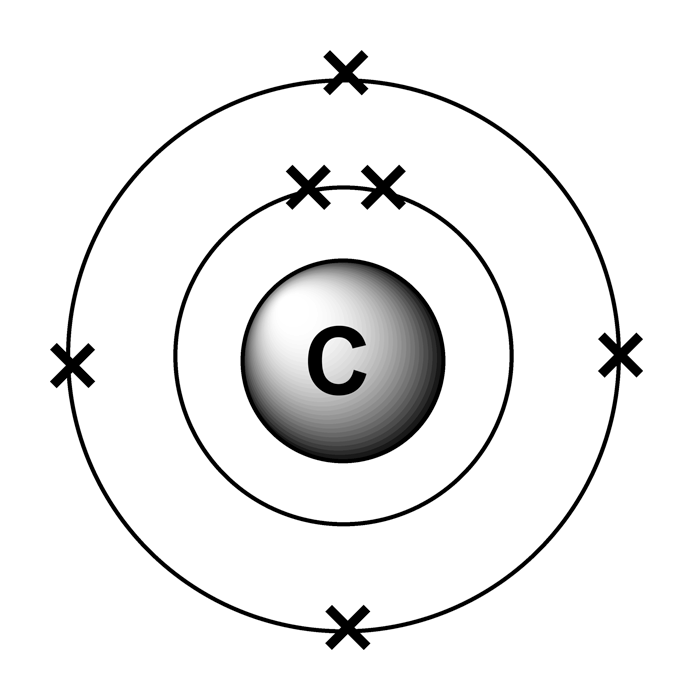
Carbon has 2 electrons in its first shell and 4 in its second shell. Calculation of valence electrons in co2 for carbon: Atom labels for all other elements are shown. Carbon is a member of the iva group and has four electrons in its valence shell.





![[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds](https://i2.wp.com/d1avenlh0i1xmr.cloudfront.net/small/65382287-b5ac-4ea6-8a57-1f5a107b172c/a-carbon-atom---teachoo.jpg)