How Many Covalent Bonds Does Hydrogen Form - Nonmetal atoms frequently form covalent bonds with other nonmetal atoms.
How Many Covalent Bonds Does Hydrogen Form - Web each hydrogen atom feels the effect of the two electrons; Methane, (\(\ce{ch4}\), is a single carbon atom covalently. Web carbon will form four covalent bonds, nitrogen will form three covalent bonds, oxygen will form two covalent bonds, and hydrogen will form one covalent. Web types of chemical bonds including covalent, ionic, and hydrogen bonds and london dispersion forces. How many covalent bonds does carbon form if each of its unpaired electrons participate in one bond?
Methane, (\(\ce{ch4}\), is a single carbon atom covalently. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and carbon makes 4 bonds. The maximum number of covalent. Only one, the one at the very top which is attached to the highly. This is because the oxygen atom, in addition to forming bonds. Web the number refers to the number of bonds each of the element makes:
Hydrogen Bond Definition, Types, and Examples
Web for example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two hydrogen atoms. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web the number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. Only one, the.
Chemical Bonds · Anatomy and Physiology
Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and carbon makes 4 bonds. Web types of chemical bonds including covalent, ionic, and hydrogen bonds and london dispersion forces. Web carbon will form four covalent bonds, nitrogen will form three covalent bonds, oxygen will form two covalent bonds, and hydrogen will form.
The top panel in this figure shows two hydrogen atoms sharing two
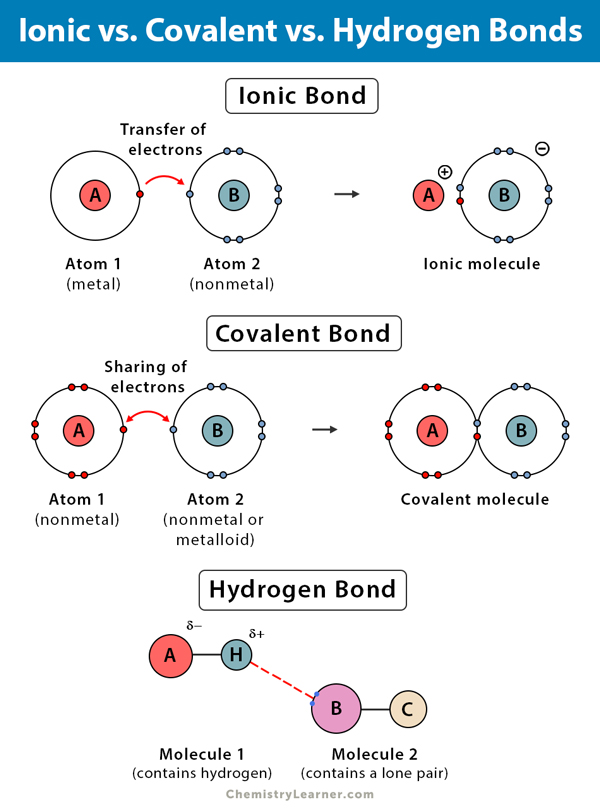
Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons; Web types of chemical bonds including covalent, ionic, and hydrogen bonds and london dispersion forces. Ad browse & discover thousands of science book titles, for less. Web the number of bonds an element forms.
Hydrogen Bonds — Overview & Examples Expii
Web although hydrogen has just one electron, it naturally forms h2, when two hydrogen atoms are held together by two unpaired electrons, forming a covalent bond. Ammonia, (\(\ce{nh3}\), is a central nitrogen atom bonded to three hydrogen atoms. Web substances with covalent bonds often form molecules with low melting and boiling points, such as hydrogen.
PPT Covalent bonding in hydrogen PowerPoint Presentation, free
A covalent bond is formed. Web although hydrogen has just one electron, it naturally forms h2, when two hydrogen atoms are held together by two unpaired electrons, forming a covalent bond. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web how many covalent bonds does hydrogen form if. Web each hydrogen atom feels.
PPT Covalent bonding in hydrogen PowerPoint Presentation, free
Living things are made up of atoms, but in most cases,. The number of bonds an element forms in a covalent compound is determined by the number of electrons it. Ad browse & discover thousands of science book titles, for less. These substances have strong covalent bonds within the. For example, the hydrogen molecule, [latex]\ce{h2}[/latex],.
PPT Covalent bonding in hydrogen PowerPoint Presentation, free
Web the number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. How many covalent bonds does carbon form if each of its unpaired electrons participate in one bond? Such a bond is weaker than an ionic bond or. Nonmetal atoms frequently form.
Question Video Determining the Number of Covalent Bonds That Hydrogen
For example, the hydrogen molecule, [latex]\ce{h2}[/latex], contains a. Web substances with covalent bonds often form molecules with low melting and boiling points, such as hydrogen and water. Each has, in a way, filled its valence energy level. The number of bonds an element forms in a covalent compound is determined by the number of electrons.
covalent bond Definition, Properties, Examples, & Facts Britannica
Web oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen forms one single covalent bond. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web formation of covalent bonds. The maximum number of covalent. Web a covalent bond is formed between two atoms by sharing electrons. So let's say.
PPT Chapter 2 The Chemical Context of Life PowerPoint Presentation
The maximum number of covalent. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web carbon will form four covalent bonds, nitrogen will form three covalent bonds, oxygen will form two covalent bonds, and hydrogen will form one covalent. Ad browse & discover thousands of science book titles, for less. This is because the.
How Many Covalent Bonds Does Hydrogen Form Web substances with covalent bonds often form molecules with low melting and boiling points, such as hydrogen and water. Ad browse & discover thousands of science book titles, for less. Each has, in a way, filled its valence energy level. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web for example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two hydrogen atoms.
The Number Of Bonds An Element Forms In A Covalent Compound Is Determined By The Number Of Electrons It.
How many valence electrons does the oxygen atom (o). Web formation of covalent bonds. Web oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen forms one single covalent bond. Web each hydrogen atom feels the effect of the two electrons;
This Is Because The Oxygen Atom, In Addition To Forming Bonds.
Web for example, water, (\(\ce{h2o}\)), has two covalent bonds between a single oxygen atom and two hydrogen atoms. Web the number refers to the number of bonds each of the element makes: Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web substances with covalent bonds often form molecules with low melting and boiling points, such as hydrogen and water.
Web Formation Of Covalent Bonds.
Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Living things are made up of atoms, but in most cases,. Each has, in a way, filled its valence energy level. Web hydrogen bonding, interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons;
Web In Water A Molecule, Hydrogen And Oxygen Atoms Share A Pair Of Electrons To Form Covalent Bonds.
Web carbon will form four covalent bonds, nitrogen will form three covalent bonds, oxygen will form two covalent bonds, and hydrogen will form one covalent. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. For example, the hydrogen molecule, h 2, contains a covalent bond. Web the number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet.