Draw The Lewis Structure For The Bromine Difluoride Ion - Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom.
Draw The Lewis Structure For The Bromine Difluoride Ion - If the molecule exhibits resonance, draw only one (1) of the resonance forms. Web drawing the lewis structure for brf 2. Determine the total number of electrons in the valence shells of bromine atoms. Each h atom (group 1) has 1 valence electron, and the o atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom.
Web how to draw lewis structure for bromine. This problem has been solved! There are 2 lone pairs on the bromine atom (br) and 3 lone pairs on all the four fluorine atoms (f). [2] hence, the valence electrons present in fluorine is 7 (see below image). The following procedure can be used to draw lewis structure for simple molecules. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. The bromine molecule contains only one element.
Lewis structure Bromine pentafluoride Sulfur tetrafluoride Xenon
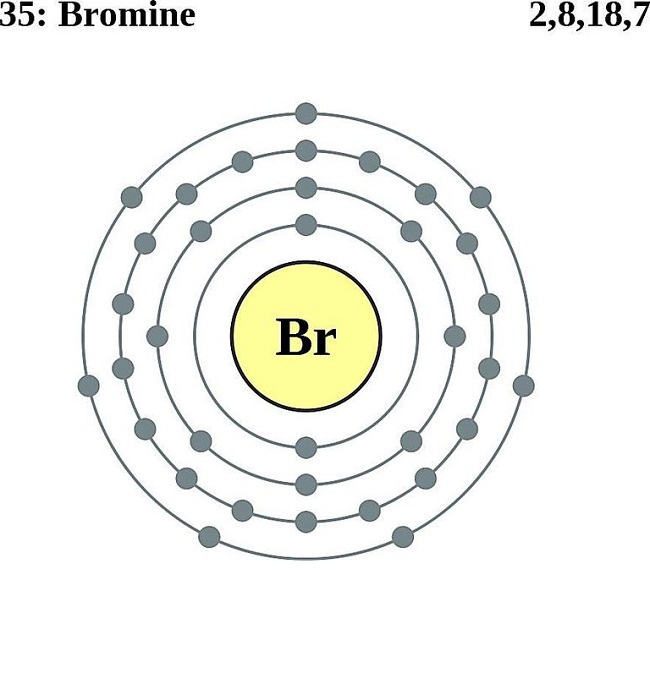
Web [1] hence, the valence electrons present in bromine is 7 (see below image). See an example of a molecule that violates the octet rule (xef₂) and learn how to draw its lewis diagram in this video. There are a few steps that need to be followed to attain the stable and correct lewis structure.
Bromine Lewis Dot Structure Drawing, Several Compounds and Detailed
Web draw lewis structures for covalent compounds. [2] hence, the valence electrons present in fluorine is 7 (see below image). The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web draw lewis structures for covalent compounds. There are 2 lone pairs on the.
Bromine Formula \({\rm{B}}{{\rm{r}}_2}\) Structure, Molar Mass, IUPAC
Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). The bromine molecule contains only one element. There are a few steps that need to be followed to attain the stable and correct lewis structure which are as follows: Valence electron, hybridization,.
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy
See an example of a molecule that violates the octet rule (xef₂) and learn how to draw its lewis diagram in this video. So, if you are ready to go with these 5 simple steps, then let’s dive right into it! Web brf2 lewis structure is drawn depending on the valence electrons of br and.
How to Draw the Lewis Dot Structure for BrF2 YouTube
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The following procedure can be used to draw lewis structure for simple molecules. If the molecule exhibits resonance, draw only one (1) of the resonance forms. This means that the central bromine (br) atom will have an odd number.
BrF2 Lewis Structure How to Draw the Lewis Structure for Bromine
Web draw the lewis dot structure of the triiodide ion , and explain what conditions /reactants are necessary for it to form this problem has been solved! There are a few steps that need to be followed to attain the stable and correct lewis structure which are as follows: Brf2 is also termed as bromo.
Solved Draw the Lewis structure of the bromine difluoride
The bromine molecule contains only one element. Valence electron, hybridization, solubility, covalent nature and polarity of brf2 is also explained. Determine the total number of valence electrons in the molecule or ion. For the brf2 structure use the periodic table to find the total number of valence electrons. Lewis structures are representations of molecules that.
Lewis Dot Diagram For Bromine Wiring Diagram
Web several lewis structures can be written for perbromate ion, , the central br with all single br—o bonds, or with one, two, or three br=o double bonds. Determine the total number of valence electrons in the molecule/ion. See an example of a molecule that violates the octet rule (xef₂) and learn how to draw.
Bromine Facts, Symbol, Discovery, Properties, Uses
Web draw the lewis structure for the bromine difluoride (brf2) ion. Web by supriya upadhya we will discuss about drawing lewis structure of brf 2, resonance, shape, formal charge, angle, octet rule, lone pairs of brf2 lewis structure. Web draw lewis structures for covalent compounds. For the brf2 structure use the periodic table to find.
BrF3 Lewis Structure (Bromine Trifluoride) YouTube
Draw the lewis structure of the bromine difluoride cation, brf2+ do not include brackets or formal charges. There are 4 single bonds between the bromine atom (br) and each fluorine atom (f). Web draw lewis structures for covalent compounds. With brf 2 there are an odd number of valence electrons (21 total). Brf2 is also.
Draw The Lewis Structure For The Bromine Difluoride Ion Valence electron, hybridization, solubility, covalent nature and polarity of brf2 is also explained. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Steps for writing lewis structures. There are a few steps that need to be followed to attain the stable and correct lewis structure which are as follows: Web drawing lewis structures for molecules with one central atom:
There Are 2 Lone Pairs On The Bromine Atom (Br) And 3 Lone Pairs On All The Four Fluorine Atoms (F).
With brf 2 there are an odd number of valence electrons (21 total). Determine the total number of valence electrons in the molecule/ion. Web by supriya upadhya we will discuss about drawing lewis structure of brf 2, resonance, shape, formal charge, angle, octet rule, lone pairs of brf2 lewis structure. See an example of a molecule that violates the octet rule (xef₂) and learn how to draw its lewis diagram in this video.
Draw The Lewis Structure Of The Bromine Difluoride Cation, Brf2+ Do Not Include Brackets Or Formal Charges.
Bromine can hold more than 8 valence electrons since it is. Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). So, if you are ready to go with these 5 simple steps, then let’s dive right into it! Determine the total number of valence electrons in the molecule or ion.
Figure Out How Many Electrons The Molecule Must Have, Based On The Number Of Valence Electrons In Each Atom.
Web several lewis structures can be written for perbromate ion, , the central br with all single br—o bonds, or with one, two, or three br=o double bonds. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web how to draw lewis structure for bromine. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
This Problem Has Been Solved!
Web drawing the lewis structure for brf 2. [2] hence, the valence electrons present in fluorine is 7 (see below image). For the brf2 structure use the periodic table to find the total number of valence electrons. Web draw lewis structures for covalent compounds.