Can Serine Form Hydrogen Bonds - Hydrogen bonding forms between a highly electronegative oxygen atom or a nitrogen atom and a hydrogen atom attached to.
Can Serine Form Hydrogen Bonds - While the sidechain is electrically neutral, this. Answer only one, the one at the very top which is attached to the highly electrongative. Polar (uncharged) serine differs from alanine in that one of the methylenic hydrogens is. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Four hydrogen atoms in the compound can form hydrogen bonds.
Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Below is the structure of the amino acid, serine. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Hydrogen bonding forms between a highly electronegative oxygen atom or a nitrogen atom and a hydrogen atom attached to. Web however, serine, by nature, is highly polar owing to its sidechain hydroxyl, with a log 10 p o/w of around −5. Polar (uncharged) serine differs from alanine in that one of the methylenic hydrogens is. One of the most useful manners by which to classify the standard (or common) amino acids is based on the polarity (that is, the distribution of electric charge) of the r group (e.g., side chain).
Hydrogen bond diagrams of functional nests. (a) In serine proteases
Web water as a perfect example of hydrogen bonding. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. (l1) is stable, that the sc(q1) → mc(l1) bond can form reversibly, and that.
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
Hydrogen bonding forms between a highly electronegative oxygen atom or a nitrogen atom and a hydrogen atom attached to. One of the most useful manners by which to classify the standard (or common) amino acids is based on the polarity (that is, the distribution of electric charge) of the r group (e.g., side chain). Web.
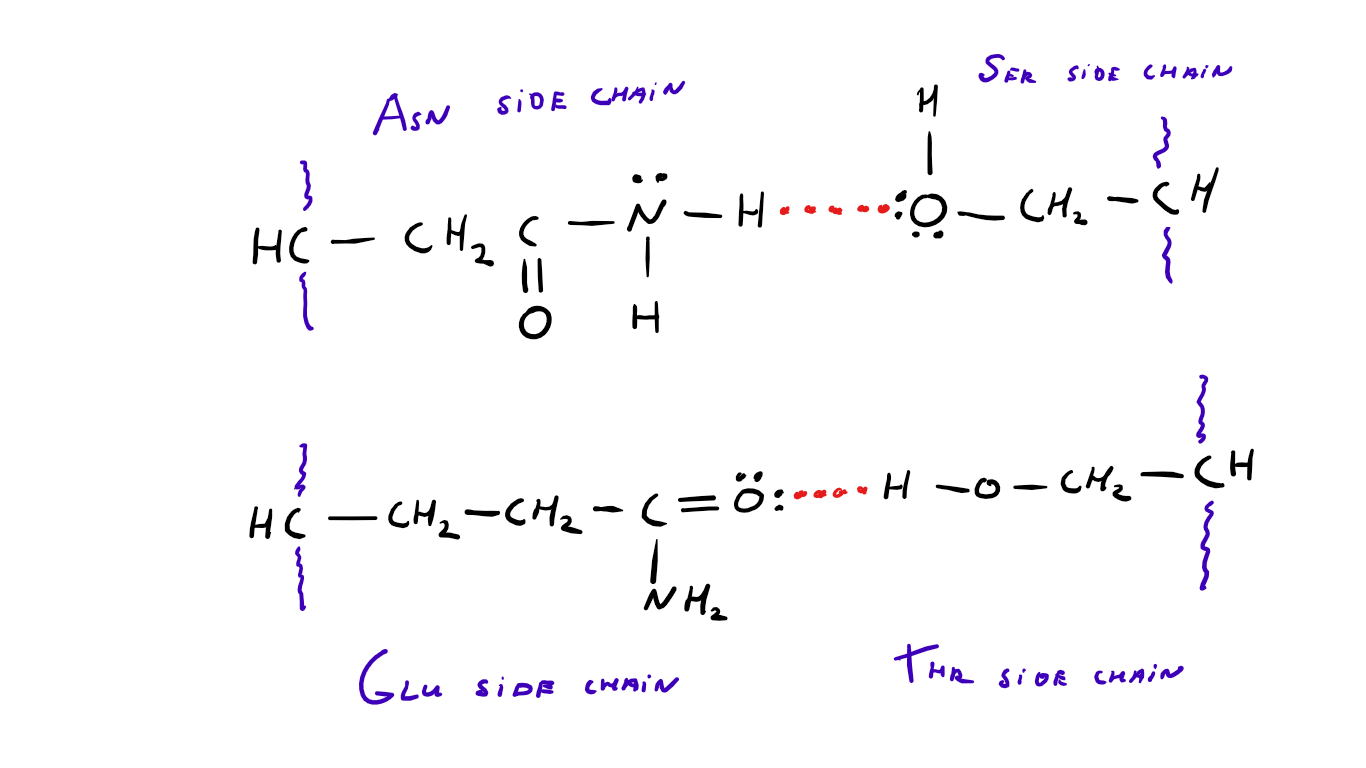
molecular structure Why do AsnSer and GlnThr have different H
While the sidechain is electrically neutral, this. (l1) is stable, that the sc(q1) → mc(l1) bond can form reversibly, and that its breakage is caused by. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web however, serine,.
Zwitterions of Lserine in forms I (a), II (b), III (c). Hydrogen
One of the most useful manners by which to classify the standard (or common) amino acids is based on the polarity (that is, the distribution of electric charge) of the r group (e.g., side chain). Web water as a perfect example of hydrogen bonding. Notice that each water molecule can potentially form four hydrogen bonds.
Rules for Hydrogen bonding? Mcat
(l1) is stable, that the sc(q1) → mc(l1) bond can form reversibly, and that its breakage is caused by. Furthermore, this group can form a hydrogen bond with. Four hydrogen atoms in the compound can form hydrogen bonds. While the sidechain is electrically neutral, this. Polar (uncharged) serine differs from alanine in that one of.
amino acids salt bridge vs hydrogen bond Chemistry Stack Exchange
A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. Polar (uncharged) serine differs from alanine in that one of the methylenic hydrogens is. (l1) is stable, that the sc(q1) → mc(l1) bond can form reversibly, and that its breakage is caused by. The most common bond arrangement.
PPT Amino Acids, Peptides, and Proteins PowerPoint Presentation, free
A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. Web however, serine, by nature, is highly polar owing to its sidechain hydroxyl, with a log 10 p o/w of around −5. One of the most useful manners by which to classify the standard (or common) amino acids.
Zwitterions of Lserine in forms I (a), II (b), III (c). Hydrogen
While the sidechain is electrically neutral, this. Furthermore, this group can form a hydrogen bond with. Web backbone carbonyls form bifurcated hydrogen bonds. Web water as a perfect example of hydrogen bonding. One of the most useful manners by which to classify the standard (or common) amino acids is based on the polarity (that is,.
Quantum chemical study of hydrogenbonded complexes of serine with
Four hydrogen atoms in the compound can form hydrogen bonds. Furthermore, this group can form a hydrogen bond with. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. (l1) is stable, that the sc(q1) → mc(l1) bond can form reversibly, and that its breakage is caused by. The most.
Serine substitutions in proteins Flickr Photo Sharing!
Four hydrogen atoms in the compound can form hydrogen bonds. The most common bond arrangement is a four. Web however, serine, by nature, is highly polar owing to its sidechain hydroxyl, with a log 10 p o/w of around −5. While the sidechain is electrically neutral, this. Polar (uncharged) serine differs from alanine in that.
Can Serine Form Hydrogen Bonds (l1) is stable, that the sc(q1) → mc(l1) bond can form reversibly, and that its breakage is caused by. Answer only one, the one at the very top which is attached to the highly electrongative. Hydrogen bonding forms between a highly electronegative oxygen atom or a nitrogen atom and a hydrogen atom attached to. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. The most common bond arrangement is a four.
Polar (Uncharged) Serine Differs From Alanine In That One Of The Methylenic Hydrogens Is.
Furthermore, this group can form a hydrogen bond with. Web however, serine, by nature, is highly polar owing to its sidechain hydroxyl, with a log 10 p o/w of around −5. Hydrogen bonding forms between a highly electronegative oxygen atom or a nitrogen atom and a hydrogen atom attached to. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds?
Notice That Each Water Molecule Can Potentially Form Four Hydrogen Bonds With Surrounding Water Molecules.
Answer only one, the one at the very top which is attached to the highly electrongative. Web water as a perfect example of hydrogen bonding. Below is the structure of the amino acid, serine. While the sidechain is electrically neutral, this.
Web Backbone Carbonyls Form Bifurcated Hydrogen Bonds.
The most common bond arrangement is a four. One of the most useful manners by which to classify the standard (or common) amino acids is based on the polarity (that is, the distribution of electric charge) of the r group (e.g., side chain). A survey of known protein structures reveals that approximately 70% of serine residues and at least 85%. (l1) is stable, that the sc(q1) → mc(l1) bond can form reversibly, and that its breakage is caused by.