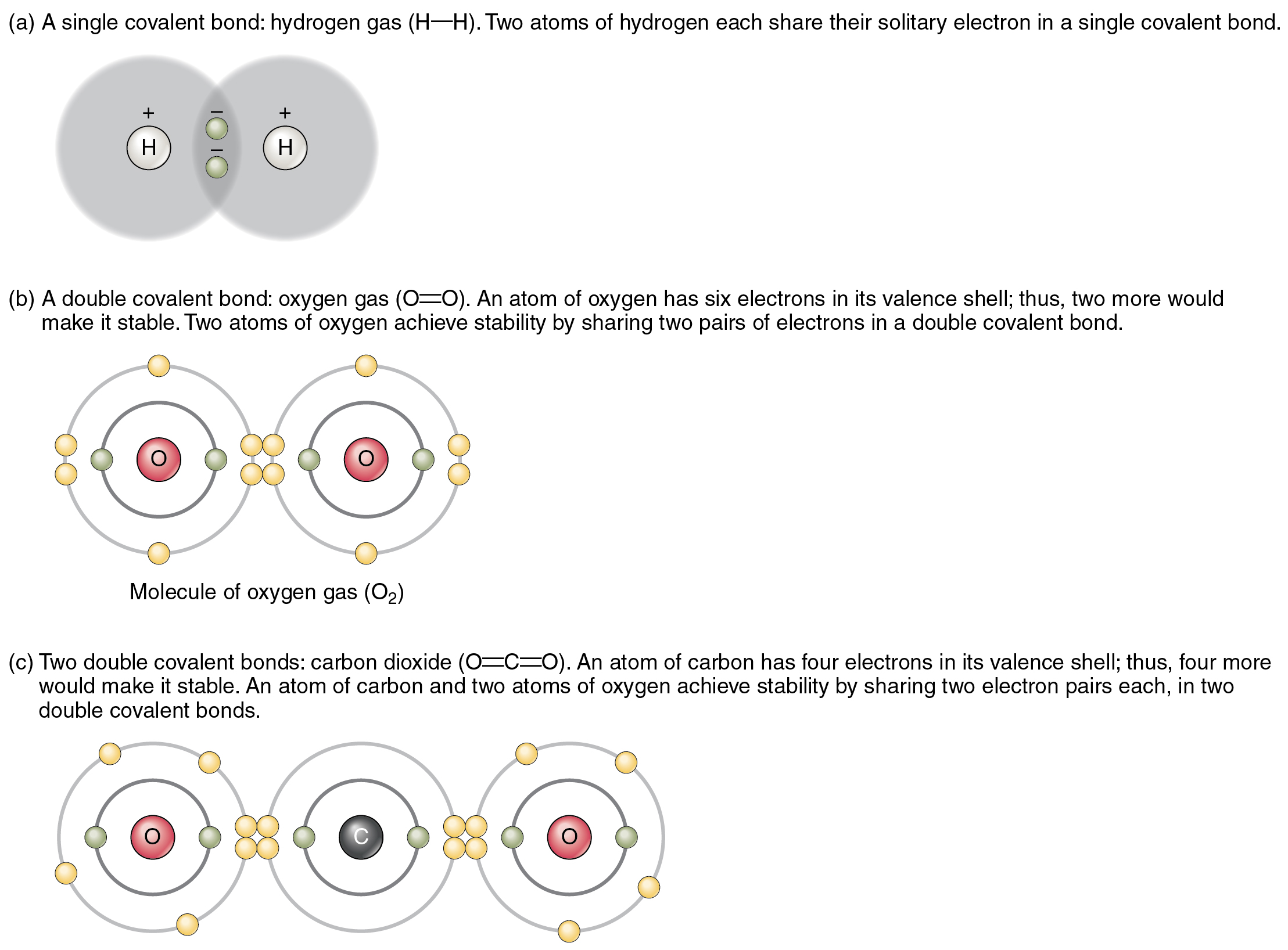
Can Carbon Form Ionic Bonds - Web when bonds are formed, energy is released and the system becomes more stable.
Can Carbon Form Ionic Bonds - Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. If carbon forms 4 bonds rather than 2, twice as much energy is released and so. Some atoms become more stable by gaining or losing an entire electron (or several electrons). The most common type of bond formed by carbon is a covalent bond. To form ionic bonds, carbon molecules must either gain or.
Atoms interact with each other through the formation of chemical bonds. Web when bonds are formed, energy is released and the system becomes more stable. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. The textbook also mentions that a. Ionic bonds result from the attraction. Web there are ionic carbides ($\ce{al4c3}$, $\ce{cac2}$, etc.), graphite intercalation compounds like $\ce{kc8}$, ionic derivatives of fullerenes and more. Carbon most often forms a covalent bond with other atoms.
Carbon Oxygen Triple Bond DaytonknoeMoses
To form ionic bonds, carbon molecules must either gain or. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Less commonly, carbon forms ionic bonds. Ad over 27,000 video lessons and other resources, you're guaranteed to find what you.
Four covalent bonds. Carbon has four valence electrons and here a
Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). If carbon forms 4 bonds rather than 2, twice as much energy is released and so. One type of chemical bond is an ionic bond. To form.
Ppt Atomic Structure And Chemical Bonding Powerpoint Presentation 7F5
Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. In general bonds of carbon with other elements are covalent bonds. Some atoms become more stable by gaining or losing an entire electron (or several electrons). Web aluminum and carbon react to form an ionic compound. This is because.
Ionic Bonding Presentation Chemistry
Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. Ad over 27,000 video lessons and other resources, you're guaranteed to find what you need. To form ionic bonds, carbon molecules must either gain or lose 4 electrons. Web moreover, of all the elements in the second row, carbon.
Ionic Bond Definition, Types, Properties & Examples
Web organic carbon compounds are far more numerous than inorganic carbon compounds. Web ions and ionic bonds. Web most chemists agree that an electronegativity difference greater than 1.7 renders a bond ionic. Ionic bonds result from the attraction. Less commonly, carbon forms ionic bonds. To form ionic bonds, carbon molecules must either gain or lose.
The 4 Types of Bonds Carbon Can Form Video & Lesson Transcript
Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Figure 2.29 some elements exhibit a regular pattern of ionic charge. One type of chemical bond is an ionic bond. Ad over 27,000 video lessons and other.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
This is because carbon has 4 valence. To form ionic bonds, carbon molecules must either gain or lose 4 electrons. Carbon most often forms a covalent bond with other atoms. If it is with another atom, a polar covalent bond is formed. Web we would like to show you a description here but the site.
PPT Unit 1 Biochemistry The Chemistry of Life PowerPoint
The most common type of bond formed by carbon is a covalent bond. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Web most chemists agree that an electronegativity difference greater than 1.7 renders a bond ionic. If carbon.
Carbon to Carbon Single, Double & Triple Bonds Surfguppy
In most cases, carbon shares electrons with other atoms. If carbon forms 4 bonds rather than 2, twice as much energy is released and so. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. Web when bonds are formed, energy is released and the system becomes more stable..
Chemical Bonds · Anatomy and Physiology
One type of chemical bond is an ionic bond. Web aluminum and carbon react to form an ionic compound. Less commonly, carbon forms ionic bonds. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. The most common type of bond formed by carbon is a covalent bond. The.
Can Carbon Form Ionic Bonds Web for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. So what's different for carbon? Web we would like to show you a description here but the site won’t allow us. If carbon forms 4 bonds rather than 2, twice as much energy is released and so. If it is with another atom, a polar covalent bond is formed.
Web Organic Carbon Compounds Are Far More Numerous Than Inorganic Carbon Compounds.
In general bonds of carbon with other elements are covalent bonds. Web there are ionic carbides ($\ce{al4c3}$, $\ce{cac2}$, etc.), graphite intercalation compounds like $\ce{kc8}$, ionic derivatives of fullerenes and more. To form ionic bonds, carbon molecules must either gain or lose 4 electrons. Web when bonds are formed, energy is released and the system becomes more stable.
This Is Because Carbon Has 4 Valence.
Web we would like to show you a description here but the site won’t allow us. To form ionic bonds, carbon molecules must either gain or. Ionic bonds result from the attraction. To form ionic bonds, carbon molecules must either gain or lose 4 electrons.
Web Aluminum And Carbon React To Form An Ionic Compound.
Write the symbol for each. Predict which forms an anion, which forms a cation, and the charges of each ion. Carbon most often forms a covalent bond with other atoms. Web most chemists agree that an electronegativity difference greater than 1.7 renders a bond ionic.
The Most Common Type Of Bond Formed By Carbon Is A Covalent Bond.
One type of chemical bond is an ionic bond. Web for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. If carbon forms 4 bonds rather than 2, twice as much energy is released and so. Carbon does not form ionic bonds because it has 4 valence electrons, half of an octet.




.PNG)